

Acids, Bases, and the pH Scale – Interactive Google Slides Lesson + Alien Juice Bar Virtual Lab
Drag-and-Drop Simulation, Indicators, Litmus Testing, and pH Interpretation Practice.
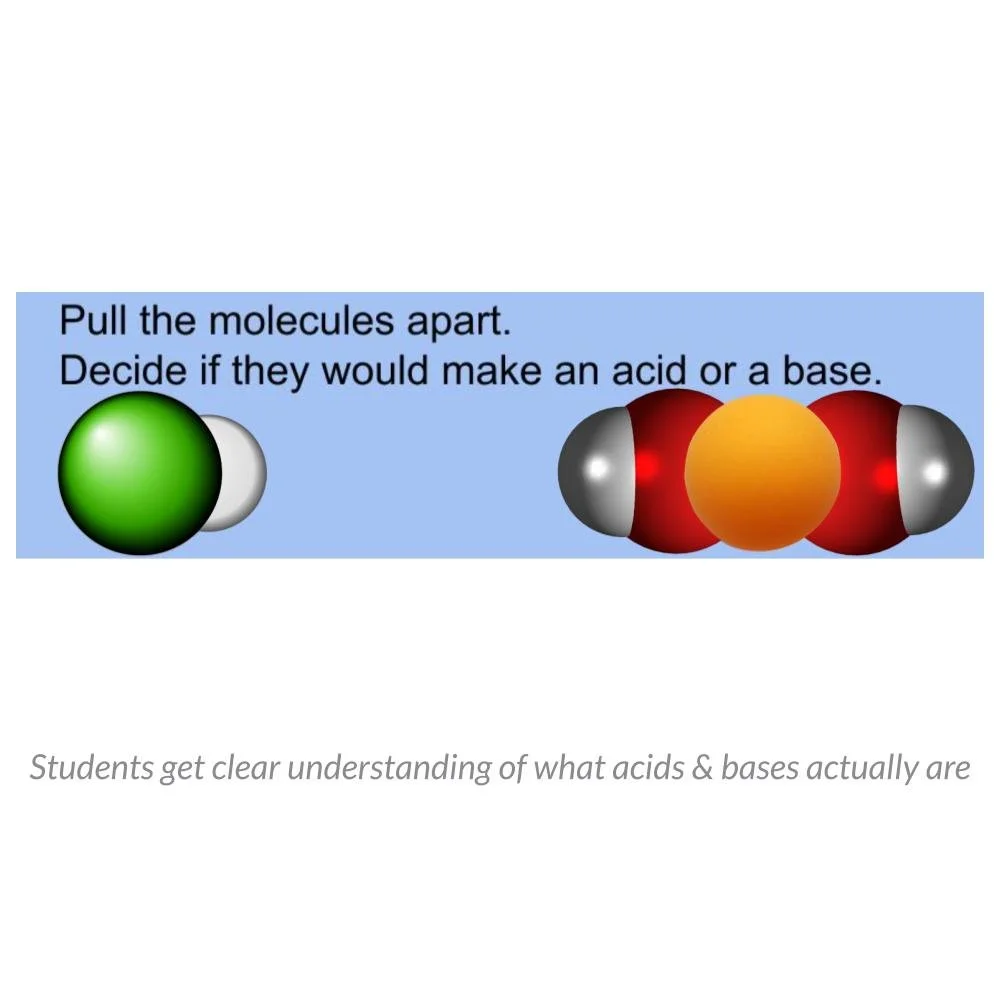
This interactive digital lesson introduces students to acids, bases, and the pH scale through molecular modeling and structured application tasks. Students work directly with particle-level representations to understand how hydrogen and hydroxide ions determine whether a substance is acidic, basic, or neutral.
The lesson is designed to move from conceptual foundations to quantitative reasoning and then to application, making it suitable for middle school and early high school chemistry or physical science courses.
What Students Do
Students begin by modeling the dissociation of water and other molecules to observe the formation of:
• hydrogen ions (H⁺)
• hydroxide ions (OH⁻)
They classify molecules based on ion production and determine whether each substance is acidic or basic.
Next, students analyze the pH scale and interpret its logarithmic structure, answering guided questions and placing values and images on a visual model of the scale.
Finally, students apply their understanding through a set of practice tasks that require them to:
• interpret pH values
• compare relative acidity and basicity
• connect molecular structure to pH behavior
Key Concepts Reinforced
• Acids vs. bases at the molecular level
• Hydrogen and hydroxide ions
• Neutral solutions
• The pH scale and logarithmic change
• Particle-level reasoning
• Conceptual chemistry modeling
Why Teachers Use This Lesson
• Visualizes abstract chemistry concepts
• Connects molecular structure to pH values
• Reinforces quantitative reasoning
• Works well as guided practice or independent work
• Suitable for digital classrooms
• Minimal prep required
Format
This lesson is delivered as a digital Google Slides activity in which students interact directly with diagrams and models.
A teacher key is included.
Best Fit For
• Middle school chemistry
• High school chemistry (introductory units)
• Physical science
• Units on acids, bases, or pH
• Classrooms introducing molecular explanations
To see a preview of this lesson, click here
Grade & Course Recommendation
Middle School: Grades 7–8 Physical Science or Life Science — ideal for introducing chemical properties and pH as part of cell processes or environmental chemistry.
High School: Grades 9–10 Biology or Chemistry — fits well into biochemistry, environmental chemistry, or laboratory safety units emphasizing acid-base interactions.
Cross-Curricular Connections
Math Integration: Students work with number scales (0–14) and logarithmic relationships to understand hydrogen ion concentration.
ELA Integration: Students write short CER (Claim–Evidence–Reasoning) responses summarizing findings from the simulation.
Technology Integration: The PhET-like interactive simulation supports visual and kinesthetic learners in modeling acid-base behavior.
Environmental Science Connection: Relates to real-world contexts such as acid rain, soil pH, and ocean acidification.
Daily slide + literacy - based exit ticket included with purchase
Join the Lesson Laboratory and Teach for Tomorrow!
NGSS Standards
NGSS
MS-PS1-2: Analyze and interpret data on the properties of substances before and after interactions to determine if a chemical reaction has occurred.
NGSS
MS-PS1-5: Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved (applies if you extend into neutralization).
NGSS
HS-PS1-3: Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles (acid/base solutions, ion interactions).
Common Core Standards
CCSS.ELA-LITERACY.RST.6-8.3/RST.9-10.3: Follow precisely a multistep procedure when carrying out experiments, taking measurements, or performing technical tasks. (connection: following steps to test pH and classify solutions as acidic, basic, or neutral)
CCSS.ELA-LITERACY.RST.6-8.4/RST.9-10.4: Determine the meaning of symbols, key terms, and other domain-specific words and phrases (e.g., acidic, basic, neutral, indicator).
CCSS.ELA-LITERACY.RST.6-8.7/RST.9-10.7: Integrate quantitative or technical information expressed in words with visual data (e.g., color changes, pH charts, or solution diagrams).
Drag-and-Drop Simulation, Indicators, Litmus Testing, and pH Interpretation Practice.
This interactive digital lesson introduces students to acids, bases, and the pH scale through molecular modeling and structured application tasks. Students work directly with particle-level representations to understand how hydrogen and hydroxide ions determine whether a substance is acidic, basic, or neutral.
The lesson is designed to move from conceptual foundations to quantitative reasoning and then to application, making it suitable for middle school and early high school chemistry or physical science courses.
What Students Do
Students begin by modeling the dissociation of water and other molecules to observe the formation of:
• hydrogen ions (H⁺)
• hydroxide ions (OH⁻)
They classify molecules based on ion production and determine whether each substance is acidic or basic.
Next, students analyze the pH scale and interpret its logarithmic structure, answering guided questions and placing values and images on a visual model of the scale.
Finally, students apply their understanding through a set of practice tasks that require them to:
• interpret pH values
• compare relative acidity and basicity
• connect molecular structure to pH behavior
Key Concepts Reinforced
• Acids vs. bases at the molecular level
• Hydrogen and hydroxide ions
• Neutral solutions
• The pH scale and logarithmic change
• Particle-level reasoning
• Conceptual chemistry modeling
Why Teachers Use This Lesson
• Visualizes abstract chemistry concepts
• Connects molecular structure to pH values
• Reinforces quantitative reasoning
• Works well as guided practice or independent work
• Suitable for digital classrooms
• Minimal prep required
Format
This lesson is delivered as a digital Google Slides activity in which students interact directly with diagrams and models.
A teacher key is included.
Best Fit For
• Middle school chemistry
• High school chemistry (introductory units)
• Physical science
• Units on acids, bases, or pH
• Classrooms introducing molecular explanations
To see a preview of this lesson, click here
Grade & Course Recommendation
Middle School: Grades 7–8 Physical Science or Life Science — ideal for introducing chemical properties and pH as part of cell processes or environmental chemistry.
High School: Grades 9–10 Biology or Chemistry — fits well into biochemistry, environmental chemistry, or laboratory safety units emphasizing acid-base interactions.
Cross-Curricular Connections
Math Integration: Students work with number scales (0–14) and logarithmic relationships to understand hydrogen ion concentration.
ELA Integration: Students write short CER (Claim–Evidence–Reasoning) responses summarizing findings from the simulation.
Technology Integration: The PhET-like interactive simulation supports visual and kinesthetic learners in modeling acid-base behavior.
Environmental Science Connection: Relates to real-world contexts such as acid rain, soil pH, and ocean acidification.
Daily slide + literacy - based exit ticket included with purchase
Join the Lesson Laboratory and Teach for Tomorrow!
NGSS Standards
NGSS
MS-PS1-2: Analyze and interpret data on the properties of substances before and after interactions to determine if a chemical reaction has occurred.
NGSS
MS-PS1-5: Develop and use a model to describe how the total number of atoms does not change in a chemical reaction and thus mass is conserved (applies if you extend into neutralization).
NGSS
HS-PS1-3: Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles (acid/base solutions, ion interactions).
Common Core Standards
CCSS.ELA-LITERACY.RST.6-8.3/RST.9-10.3: Follow precisely a multistep procedure when carrying out experiments, taking measurements, or performing technical tasks. (connection: following steps to test pH and classify solutions as acidic, basic, or neutral)
CCSS.ELA-LITERACY.RST.6-8.4/RST.9-10.4: Determine the meaning of symbols, key terms, and other domain-specific words and phrases (e.g., acidic, basic, neutral, indicator).
CCSS.ELA-LITERACY.RST.6-8.7/RST.9-10.7: Integrate quantitative or technical information expressed in words with visual data (e.g., color changes, pH charts, or solution diagrams).